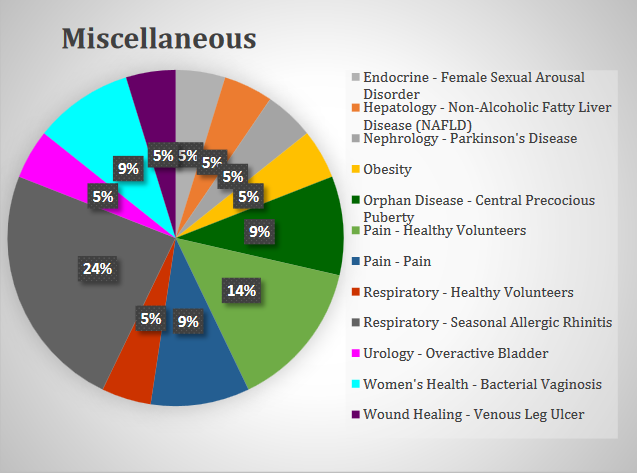
Miscellaneous
- Central Nervous System
- Healthy Patient Experience
- Hepatology
- Oncology
- Orphan Disease
- Regenerative Medicine
- Respiratory
- Urology
- Women’s Health
- Wound Healing
Integrium’s Central Nervous System Clinical Trial Experience
| Overall CNS Experience | ||||
|---|---|---|---|---|
| Study Phase | Indication | # Patients | # Sites | Location |
| l | Parkinson’s Disease | 40 | 4 | US |
| ll | Neurogenic Orthostatic Hypotension |
30 | 25 | US |
| lll | Stroke | 1822 | 220 | US |